INSelN - Morphogenic Impact of Inhibitory Neurons in Self-organizing Networks
Project Description
The architecture of the mammalian neocortex and other brain regions is not exclusively based on the execution of genetic programs but strongly relies on the homeostatic regulation of neuronal activity guiding the structural and functional differentiation of neurons and networks.
The maturation of inhibition during the activity-dependent wiring process implies complex developmental interactions between excitatory and inhibitory neurons that are poorly understood so far. This project will utilize generic networks in vitro and in silico (Fig. 1) to address the following question herein:
Is the activity-dependent interaction of neuronal migration, neurite outgrowth and maturation of inhibition influencing the spatial distribution of excitatory and inhibitory neurons and their inter-connectivity?
Is the activity-dependent structural differentiation of neurons contributing to aspects of excitation and inhibition balance within local circuits or at the network level?
Is maturing inhibition promoting modular network architectures that are considered beneficial for stable activity dynamics and neuronal computation?
Close collaboration between an experimental and a theoretical PhD study is aimed at dissecting how network activity and the morphological respectively functional development of inhibition shape a network’s structure in a homeostatic loop. Versatile experimental techniques will be used to investigate and manipulate the development of inhibition and network architecture in vitro. In turn, computational modelling will allow to generate specific hypotheses and expectations for different developmental scenarios that can be tested and constrained experimentally.
Conjointly, both approaches will work towards a general mechanistic understanding of the role of maturing inhibition in the activity-dependent structural and functional development of neuronal networks.
This projected is funded by the German Research Foundation (DFG, project number: 516753473).
Please note that do not have any open position.
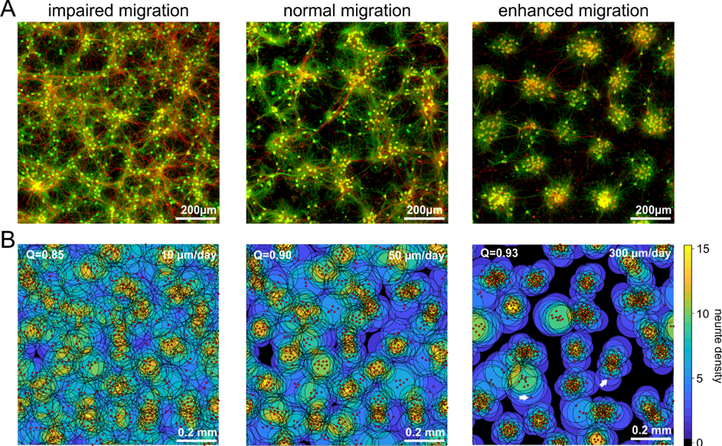
Fig. 1) Manipulation of neuronal motility and migration during the activity-dependent network development in vitro (A) and in silico (B). Impaired neuronal migration results in more homogeneous networks with larger inter-neuron distances and larger neurite fields established by neurons to satisfy their need of synaptic input. Enhanced neuronal migration, in turn, results in strong clustering of neuronal somata and small neurite fields reflecting increased local availability of synaptic partners. The topological and functional consequences of increasing clustering and activity-dependent down-regulation of neurite-fields are increasing network modularity and gradual desynchronization of network activity.
Reference:
Okujeni S, Egert U (2019). Self-organization of modular network architecture by activity-dependent neuronal migration and outgrowth. eLife 8:e47966